PLC Systems Receives FDA Approval to Commence Pivotal Study of RenalGuard® in the U.S.
Modified design builds upon successful international studies
Wed Oct 19 2011
MILFORD, Mass., Oct. 14, 2011 -- PLC Systems Inc. (OTC: PLCSF), a company focused on innovative medical device technologies, announced today that it has received final approval from the U.S. Food and Drug Administration (FDA) to commence its U.S. pivotal trial to study the efficacy of the Company's RenalGuard Therapy® and RenalGuard System(TM) in the prevention of Contrast-Induced Nephropathy (CIN).
Mark R. Tauscher, President and Chief Executive Officer of PLC, said, "We've very pleased to be able to move forward with our U.S. pivotal trial, starting with many of the hospitals that had participated in our earlier trial. Using lessons learned from the clinical trials of RenalGuard® in Europe as well as other clinical developments since 2008, we made slight modifications to our original trial protocol that we believe will make it more scientifically significant. We are now finalizing our submissions to the hospital Institutional Review Boards and will begin enrolling patients in the near future. This is the first step on our path forward to seeking FDA approval to market the RenalGuard therapy and system in the U.S."
PLC's U.S. pivotal study is under the supervision of Principal Investigators Charles Davidson, MD, Professor of Medicine, Northwestern University Medical School, Richard J. Solomon, MD, Professor of Medicine, University of Vermont College of Medicine and Roxana Mehran, MD, Professor of Medicine at Mount Sinai School of Medicine. It is designed as an adaptive, randomized controlled trial at up to 30 sites in the U.S. Enrollment in the trial will include at least 326 patients and potentially up to 652 patients, depending upon the outcome of a sample size re-estimation after 163 patients. The sample size re-estimation, often used in adaptive trials, enables investigators to ensure that the trial is sufficiently powered so that the final results are statistically meaningful.
PLC's U.S. study builds upon two clinical trials by independent clinical investigators in Europe, both of which showed significant reductions in incident rates of CIN in at-risk patients through the use of RenalGuard compared to the current standard of care.
Contrast-Induced Nephropathy
CIN is a major and growing problem due to the increasing number of older patients, diabetics and patients with pre-existing renal impairment - all of whose conditions make them at risk for CIN when they require interventional procedures that use radiographic contrast media. More than 7 million such imaging procedures occur worldwide each year, and it is estimated that 15-20% of those patients are 'at risk' of acquiring CIN. CIN is the third most common cause of in-hospital acute renal failure. It is associated with significant in-hospital mortality rates, and increases in long-term mortality rates, major in-hospital adverse cardiac events, and increased risk of renal dialysis therapy. All of these lead to prolonged hospital stays and increased medical costs. Estimated mortality rate for patients who acquire CIN may be as high as 35%.
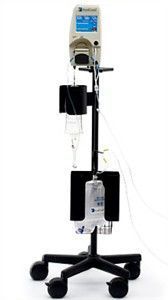
About RenalGuard
RenalGuard is based on data that shows that initiating and maintaining high urine output during imaging procedures allows the body to rapidly eliminate toxins in contrast media, reducing their harmful effects. RenalGuard is a fully-automated, real-time matched fluid replacement device intended for interventional cardiology and radiology patients undergoing imaging procedures using contrast media.
About PLC Systems Inc.
PLC Systems Inc., headquartered in Milford, Mass. , is a medical device company focused on innovative technologies for the cardiac and vascular markets. PLC's newest product, RenalGuard, has been developed to help prevent the onset of Contrast-Induced Nephropathy (CIN) in at-risk patients undergoing certain imaging procedures. The Product is CE-marked and is being marketed in Europe and selected countries around the world. Two investigator-sponsored European studies have demonstrated RenalGuard's effectiveness at preventing CIN. RenalGuard is being studied in a pivotal trial in the U.S., as required for approval by FDA, and has not been approved for patient use in the United States.
Additional company information can be found at www.plcmed.com.
This press release contains "forward-looking" statements. For this purpose, any statements contained in this press release that relate to prospective events or developments are deemed to be forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "will" and similar expressions are intended to identify forward-looking statements. Our statements of our objectives are also forward-looking statements. While we may elect to update forward-looking statements in the future, we specifically disclaim any obligation to do so, even if our estimates change, and you should not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release. Actual results could differ materially from those indicated by such forward-looking statements as a result of a variety of important factors, including that we may not receive necessary regulatory approvals to market our RenalGuard product or that such approvals may be withdrawn, the U.S. clinical trial for RenalGuard may not be completed in a timely fashion, if at all, or, if this clinical trial is completed, it may not produce clinically significant or meaningful results, the RenalGuard product may not be commercially accepted, operational changes, competitive developments that may affect the market for our products, regulatory approval requirements that may affect the market for our products, and additional risk factors described in the "Forward Looking Statements" section of our Annual Report on Form 10-K for the year ended December 31, 2010, a copy of which is on file with the SEC.
PLC Systems, RenalGuard, RenalGuard Therapy and RenalGuard System are trademarks of PLC Systems Inc.
