Nihon Kohden - BSM-2300 series
by Nihon Kohden
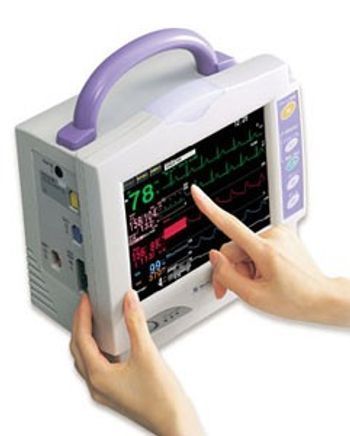

With high performance in a compact body, Life Scope i is ideal for transport monitoring and bedside monitoring for ER, OR and ICU, PACU, NICU, step-down unit, and general ward. [b][i]This product is not sold in the U.S by the manufacturer.[/i][/b]
2Replies6 years ago | circuitry scheme Nihon Kohden BSM-2300 s Can anyone help me to find or provide me a circuitry scheme of this monitor?Reply |
2Replies7 years ago | End of life When did Nihon Kohden BSM series reach end of life?Reply |
3Replies9 years ago | Cardiac monitor/patient monitor Friends,,,,How can i will change language in NIHON KOHDEN cardiac monitor BSM-2303 and BSM-4111,,, Reply |
[list] [*] Compact and lightweight with 3-hour battery operation [*] High resolution (800×600 dot) 8.4-inch TFT color LCD touch screen [*] 7 parameters - ECG (3 or 6 electrodes) - Respiration (impedance and thermistor) - SpO2 - NIBP - Temperature - IBP or CO2 (mainstream) - second IBP (BSM-2303) [*] 5 waves (BSM-2301) or 6 waves (BSM-2303) [*] MULTI connectors [*] Effective ECG monitoring [*] Nurse-oriented [*] Multigas measurement with AG-920R Multigas Unit [*] Full line-up of CO2 sensors including cap-ONE for non-intubated patients [*] OCRG screen for neonatal monitoring [*] Networking [*] Optional 3-channel recorder [*] Revolutionary PWTT technology [/list]
Resolution: 800×600 dot