
4 Questions You Need to be Able to Answer For Your Sonographers
Check out the newest Ask the Expert, written by Ted Lucidi!
Wed Mar 01 2023
The Innovatus team not only supports HTM teams and sonographers through comprehensive ultrasound probe repair, but also assists with helping them reduce support costs. The most effective means is, first, understanding WHY a probe has failed, and second, taking preventive measures to minimize future occurrences. Below are some common questions that we receive:
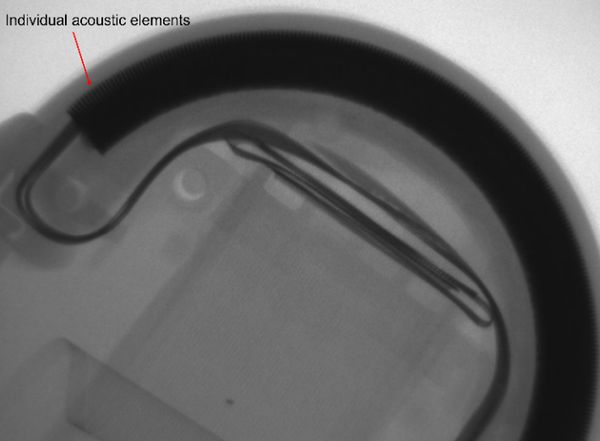 How do the “crystals”, or acoustic array, become damaged, or why do I have dropout in my image?
How do the “crystals”, or acoustic array, become damaged, or why do I have dropout in my image?
Most clinical professionals believe that the main failure of the “crystals” or acoustic array is related to performance degradation or just internal failures. Although these are valid root causes, the most common root cause is related to physical damage. Adding to the confusion for the clinical staff is the fact that, most times, there is no outward sign of trauma or physical damage to the probe housing.
The thickness of the acoustic elements or “crystals” within an acoustic array can be less than 0.5mm. The majority of acoustic arrays in ultrasound probes utilize PZT (lead zirconate titanate). To the layperson, PZT is a ceramic-like compound. What happens when your ceramic coffee mug falls to the floor? With an ultrasound probe, the force of an accidental fall to the floor is more than enough to induce significant damage to the fragile elements. The result is often dark vertical (hypoechoic) lines, or dropout, in the image.

In 2022 alone, our evaluation team performed over 10,000 image quality and performance assessments. After evaluating over a quarter-million probes, with a very high degree of confidence, our teams are able to distinguish between an acute failure of the array due to trauma, and array/electronic failures related to normal wear.
Why are probe repairs so costly?
A high percentage of costly probe failures are a result of not addressing one or more minor problems. Not addressed in a timely manner, minor physical problems can contribute to, or be the root cause of, failures that require major invasive repairs. Now, more than ever, probes are not only cleaned after every use, but exposed to harsh disinfectants. Today, normal wear includes slow and gradual degradation of the seals surrounding the acoustic lens, and separation of the seams that join the various parts of the probe housing. With a small break in physical integrity in the lens or a seam/seal on the probe housing, corrosive disinfectants gain access to the underlying acoustic array and associated electronics. It’s vital for sonographers to perform frequent visual inspections to every probe in their department, and alert HTM teams of any concerns. Addressing minor issues in a timely manner is one of the most-effective means of combating high repair costs and probe replacements.
What type of disinfectants are best for my ultrasound probe?
Today, for ultrasound probes alone, healthcare facilities have the ability to choose from over 100 different OEM-approved chemical disinfectants and even more which are not OEM-approved. Some of the most common disinfectant wipes found in healthcare facilities are Sani-Cloth products. There are at least 10 very different versions of Sani-Cloth wipes. Each has its own benefits, and each has very specific instructions for use (IFUs) and some are not indicated for probe disinfection. Key points include exposure time, application methods, and rinsing practices.
You may not know that disinfectants containing alcohols and ammonium chloride solutions can induce significant damage to plastics and rubber materials. And most importantly, you may not know that most of the disinfectants used on ultrasound probes contain these chemicals. The use of alcohols and ammonium chlorides, over time, can lead to excessive stiffness, brittleness, shrinking, and staining of the lens, strain relief, sealants, housings, cable sheathings, etc.
Typically, the choices that end-users have, for disinfectants, are limited by supply chain in combination with the infection control department. These departments may be more focused on efficacy and cost than on the effects to the medical devices on which the disinfectants are used. Even OEM-approved chemicals (and the methods in which they are used) have the potential to affect materials over time. It’s important to work with supply chain and infection control to source OEM-approved disinfectants with little to no alcohol and ammonium chloride content and be sure that end-users are following the recommended practices.
Is it not better to just replace an ultrasound probe than to repair it?
The short answer is possibly, if all that is of interest is speed, with little concern about cost. Probe replacement, whether through an OEM or third-party, may be the most efficient means of supporting ultrasound probes, but it is the least cost-effective and will typically have a much shorter warranty period than a repair from Innovatus Imaging. Innovatus understands that uptime is more important than ever, which is why we stock over 2000 loaners and can have one at your facility tomorrow, first AM. Not only is uptime critical, but overall long-term costs. Long-term costs include intangible items such as downtime, end-user satisfaction, patient satisfaction scores, product lifecycle, and quality performance. At one time, one OEM suggested that the average lifecycle of an ultrasound probe is about 3-4 years. I argue that, if repairs are performed using the correct materials and components, and the provider is using verified and validated processes, that a probe’s lifecycle could be reset multiple times. The results are increased product lifespan, restored performance, superior return on investment, and ultimately, reduced healthcare costs.
Staying ahead in healthcare technology management is all about staying informed and up to date on new developments, trends, procedures, and processes associated with current technology. Organizations which continuously engage in research and development, design, testing, verification and validation have a proven impact on efficiencies, costs, and outcomes. As an OEM for ultrasound probes as well as a premium service provider, Innovatus Imaging is continuously improving upon existing and qualifying new processes. The goal is to not just repair a device and get it working again, but to restore performance and reset the device’s lifecycle. For more information on how Innovatus can help your facility combat skyrocketing support costs within the ultrasound and MRI modalities, click here. If you have specific questions on probe performance, testing, or troubleshooting, please submit them below.
